“Is there really an endocannabinoid system?” Yes. Although not currently included in textbooks and science curriculums, the endocannabinoid system (ECS) does exist. Cannabis is a plant that happens to interact with the ECS to cause a range of effects. Since cannabis’ re-emergence into mainstream health and media, it has been under heavy political pressure from all sides; involving scheduling, classification, patient access, medicinal effects, and safety. Therefore, the following article aims to shed light on the intricate, yet mysterious system that is responsible for orchestrating the effects of cannabis, while also providing useful insight for society.
The bodily systems that receive all the attention in textbooks are the circulatory, digestive, endocrine, integumentary, immune, lymphatic, muscular, nervous, renal, reproductive, respiratory, and skeletal systems. The endocannabinoid (endo-can-na-bin-oid) system is a biological system much like the other twelve systems in the human body. Compared to the discovery of the other systems, the ECS is relatively new, with its discovery only taking place in the 1990’s. Scientists were late to the party, as phylogenetic studies (the study of evolutionary relationships) showed that the ECS had evolved over 600 million years prior to its discover and currently exists in all mammals. In fact, the ECS enables the constant balance of physiological processes; which includes pain, inflammation, sleep, learning, memory, energy, metabolism, digestion, reproduction, exercise, stress, thermoregulation, and appetite. In other words, balance is the core function of the ECS, biologically known as homeostasis. How does it manage to do all of this? To answer that, we will first need to introduce the components of the system. Until then, one can imagine the way in which the ECS operates to be synonymous to a complex audio control panel. By being able to finely tune a wide variety of outputs in response to changes in the environment.
The ECS is comprised of three different components that help take care of the aforementioned processes. Whether you have ever taken cannabis or not, these three components are working around the clock to keep physiological processes in check. They include the endocannabinoids, their metabolising enzymes, as well as the cannabinoid receptors. First off, endocannabinoids are the molecules that are naturally synthesised within your body and help control many of the key functions stated earlier. The two endocannabinoids produced in our body are Anandamide and 2-AG. Secondly, the metabolising enzymes help with the degradation of these endocannabinoids after they have fulfilled their purpose. These enzymes are called MAG-L and FAAH. Finally, the cannabinoid receptors are the structures that endocannabinoids and other molecules can bind to and broadly speaking, result in downstream effects. The two cannabinoid receptors in the body are the cannabinoid type-1 (CB1) and cannabinoid type-2 (CB2) receptors. The CB1 receptor is found in large concentrations in the brain and less so throughout the body, whereas the CB2 receptor is found in greater quantities throughout the body and less so in the brain. Collectively, their extensive coverage allows for the diversity of functions the ECS plays a role in. To illustrate this point, below is a microscopic image taken during my studies into the RNA expression of the cnr1 gene in mouse models. Depicted is a mouse brain slice that has been prepared and stained for the cnr1 gene (red), which is responsible for producing the CB1 receptor. The red dots serve as a marker, demonstrating the abundance of CB1 receptors in various areas of the brain.
Besides the endocannabinoids, anandamide and 2-AG, there are other types of cannabinoid molecules. Cannabinoids are classified depending on their source. Ultimately, cannabinoids are either endo-cannabinoids, meaning they are produced within the body or exogenous cannabinoids meaning they are produced outside of the body. Besides the endocannabinoids discussed above, exogenous cannabinoids are further classified as either phytocannabinoids or synthetic cannabinoids. Phytocannabinoid are plant-derived, like those found in cannabis. In fact, a total of 144 unique phytocannabinoids have been isolated from the cannabis plant alone, including the widely known THC and CBD phytocannabinoids. On the other hand, synthetic cannabinoids are those that are produced in a laboratory. Regardless of their source, cannabinoids, in some form or another interact with our cannabinoid receptors.
Each of us has an ECS but the capacity in which that system operates may be different. This is referred to as endocannabinoid tone. This tone can be described as the level of ECS activity. This activity is based on factors such as the extent of endocannabinoid synthesis, enzymatic activity, density of cannabinoid receptors. It also includes environmental factors such as but not limited to stress, exercise, and mood. Therefore, even though one’s endocannabinoid tone may vary from year to year or even day to day, this tone is generally unique to each of us. The variability in endocannabinoid tone helps explain the inconsistencies in response people may have to taking cannabinoids, such as those found in cannabis.
So, what exactly do our endocannabinoids do? The endocannabinoids represent brakes to the system. Essentially, they dampen the probability of neurotransmitters getting released and thus taking effect. As you may know, different neurotransmitters such as dopamine, glutamate, noradrenaline, serotonin, GABA, and glutamate all have differing effects. Therefore, based on the type of neurotransmitter whose response was dampened, the resulting effect is also changed. Generally speaking, these can be categorised into either the blocking of an excitatory signal (inhibitory effect) or blocking of an inhibitory signal (excitatory effect). Furthermore, the spatial considerations of where in the brain these neurotransmitters are or are not being released plays further into the variances in effect.
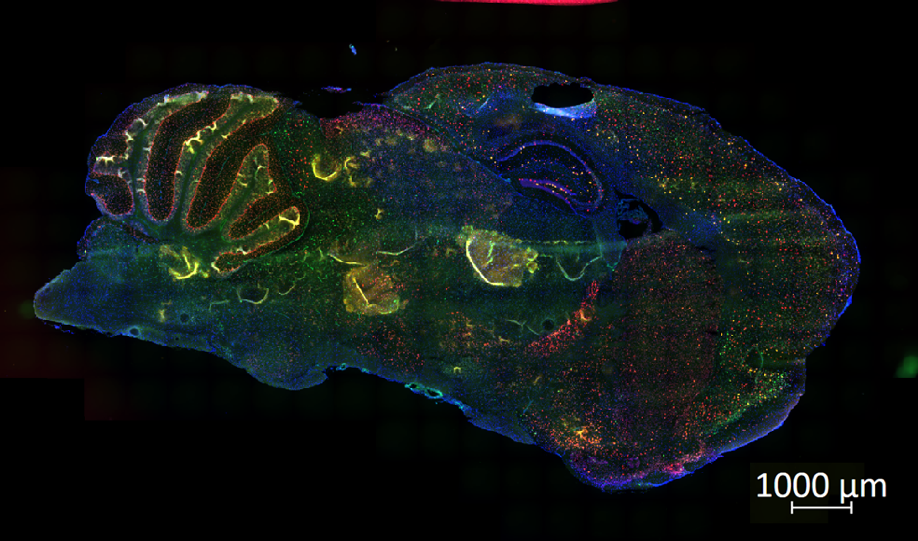
Depicted is a mouse brain slice that has been prepared and stained for the cnr1 gene (red), which is responsible for producing the CB1 receptor
Despite the large efforts of research and development (R&D), as well as clinical trials, regulatory approval of cannabinoid-based treatments are few and far between. Currently, there are four cannabinoid-based medicines approved by the US Food and Drug Administration (FDA). Three of these medications are synthetic cannabinoids indicated for either adult AIDS patients suffering from anorexia related weight loss or adult patients suffering from chemotherapy induced nausea and vomiting. These drugs are synthetic forms of THC; they include Dronabinol (Marinol, Syndros) and Nabilone (Cesamet).
The fourth medicine, demonstrates that cannabinoid-based treatments may not solely be synthetic. As of June 25th, 2018, Epidiolex (Epidyolex) became the first plant-derived cannabinoid drug to be approved by the FDA. Epidiolex is an oral solution of highly purified, plant-derived CBD approved for the treatment of two rare and highly debilitating forms of epilepsy, known as Lennox-Gastaut syndrome (LGS) and Dravet syndrome. After Epidiolex was approved by the FDA, it was classified as a Schedule I drug. This was because cannabis and anything derived from the cannabis plant was considered to be Schedule I. This included CBD, and thus implicated Epidiolex (CBD) into the same classification. Upon examination, Cannabis’ Schedule I classification was justified based on the high abuse potential and lack of medical value confirmed by standardised, double-blind, randomised control trials. What about Epidiolex? It has demonstrated the medical efficacy to warrant an FDA approval. Therefore, nonclinical and clinical studies were conducted to further investigate its abuse potential. After a few months, the Drug Enforcement Administration (DEA) reclassified Epidiolex as Schedule V, the lowest level due to its established medical use and low abuse potential.
So far, this article has examined some of the science behind the ECS and regulatory approaches to the approval and scheduling of cannabinoid-based medicines. It is time to consider the patients – because what value can a treatment truly have without patient access. There is an underlying pattern behind currently approved cannabinoid-based medications. They are last choice treatments, only considered after all other conventional therapies have failed. This has made patient access difficult, even in areas of the world that have legalised medical cannabis. For example, the United Kingdom (UK) maintained the Schedule I classification for Epidiolex, which significantly hindered patient access. Additional factors such as the stigma behind cannabis-derived medicines and lack of understanding behind the mechanisms of CBD have led health care professionals and consultants to feel uneasy when it comes to prescribing these treatments, especially given their scheduling. Fortunately, on June 24th, 2020, the British National Health Service (NHS) also down-scheduled Epidiolex to the lowest level (Schedule V) of controlled substances. This would resolve many of the aforementioned difficulties patients encountered when trying to access this medication. Although this may seem like a simple decision that should have been made long ago, regulatory bodies like the NHS and FDA have legitimate cause for concern.
This can be highlighted through the history of one such drug. As covered earlier, the ECS plays a role in behaviours like feeding and appetite. It is through this same process that cannabinoids like THC bind to the CB1 receptor and cause cravings, commonly known as the ‘munchies.’ Therefore, researchers theorised that blocking this pathway of the ECS would suppress appetite and lead to weight loss. This led to the innovation of a potential weight loss wonder-drug for obese patients. Clinical trials and research proceeded, demonstrating considerable effects in reducing both appetite and weight in animal and human models. In 2006, Europe approved Rimonabant while the FDA remained concerned about its safety profile. Two years later, it was removed from market. It was found that the antagonistic effects on the CB1 receptor not only reduced appetite but also modified other processes and functions. This led to the incidence of negative psychoactive side effects, such as depression and suicidal ideation being associated with the use of Rimonabant. With regards to the ECS-control panel example, while attempting to block or reduce the effects of one switch in the system, many other switches were also affected which throws the endocannabinoid system out of balance. Due to the complexity of the ECS, it is incredibly difficult to achieve desired outcomes without a more fine-tuned approach. This combined with the lack of knowledge regarding the mechanisms of cannabinoid action and long-term safety studies, warrants a careful and comprehensive approach to policy and regulation. Going back to the topic of Epidiolex, at the high doses indicated in such patients, CBD can cause nausea, sedation, and liver toxicity. Therefore, when it comes down to such decisions, it inevitably becomes an evaluation of whether the potential benefits outweigh the potential risks. The evaluation of potential benefits and risks can be extremely difficult, which is why research and clinical trials must continue to deepen our understanding of the endocannabinoid system and how novel molecules can maximise therapeutic benefit.
With that being said, the number of approved cannabinoid-based medicines and products will only continue to rise. Particularly because research and development around the cannabinoid space has been overly active with companies pushing their lead compounds through various stages of the developmental pipeline. In addition, the market is quickly expanding as companies are working to deliver cannabinoids via novel formulations that are more precise and deliberate in their actions. As is usually the case when it comes to dealing with uncertainty, only time will tell.
